Design and Synthesis of Integrated Nanocatalysts
|
|
|
|
|
Related
Research Thrusts
|
|
• Design and synthesis of nanomaterials for energy and
sustainability
• Development and integration of novel catalytic
materials
• Synthetic architecture of advanced functional
materials
|
|
Program
|
|
In recent years, “nanocatalysts” has become a term often
used by the materials chemistry and catalysis community
[1]. By controlling particle composition, structure,
shape, and dimension, researchers can move into the next
phase of catalyst development if they are able to bridge
old and new technologies. In this regard, one way seems
to be to integrate active catalytic components with
boundary-defined supports, which therefore retains the
essence of traditional “catalyst-plus-support”
configuration. The resultant catalysts have advantages
for material engineering that often involves high level
designs and integrations in their fabrication. Besides
this, the active components in this new type of
catalysts are in nanoscale and are easy to integrate
into supporting material phases. For these reasons, we
name such catalytic devices as integrated nanocatalysts
(INCs) [1]. In our group, we carry out this type of
materials research and develop new synthetic strategies
and architectural designs for INCs with increasing
compositional and structural complexities in order to
meet new challenges of heterogeneous catalysis in energy
and sustainability applcations.
|
|
Background
|
|
Integrated nanocatalysts (INCs) with multicomponent and
hierarchically complex structures have recently drawn
extensive research attention in terms of their
fundamental sciences and industrial applications, and
many innovative strategies have been established [1]. In
pursuing this type of research, we are developing the
state-of-the-art of INCs with different porous materials
for catalyst technology and heterogeneous catalysis. In
our research, we synthesize different nanoparticles or
clusters (namely, metal, metal oxide, and hybrid
nanoparticles) that are catalytically active in INCs
[2]. Combining such active components with various
porous materials provides us a huge array of
architectural designs for INCs that can be used in
chemical reactions. In choosing host materials for INCs,
we particularly focus on shape-controlled mesoporous
siliceous materials (e.g., silica and metal silicates)
and microporous metal-organic frameworks (MOFs), since
they represent the two important classes of porous
materials known today. In our research program,
therefore, we develop INCs which comprise these
mesoporous and microporous solids and active catalytic
components. A special emphasis is placed on their roles
as supports, encapsulating shells and metal sources for
targeted applications of energy and sustainability.
|
|
Methodologies
|
|
Basically, two types of materials are used in
construction of these catalytic devices: active catalyst
components and their supporting carriers [3-8]. In
preparing INCs, various synthetic methods have been
developed in recent years [5, 6]. Normally, the active
components are synthesized into monodisperse
nanoparticles through wet chemical routes. On the other
hand, the catalyst carriers or hosts are often prepared
as hollow or porous materials with desired
shape-controls [3, 4]. In general, integration of the
above two types of catalytic materials can be achieved
in a step-by-step manner [7-9]. Both top-down
(e.g., dissolution and regrowth) and bottom-up
(e.g., self-assembly and deposition) strategies have
been employed in the synthetic architecture of INCs
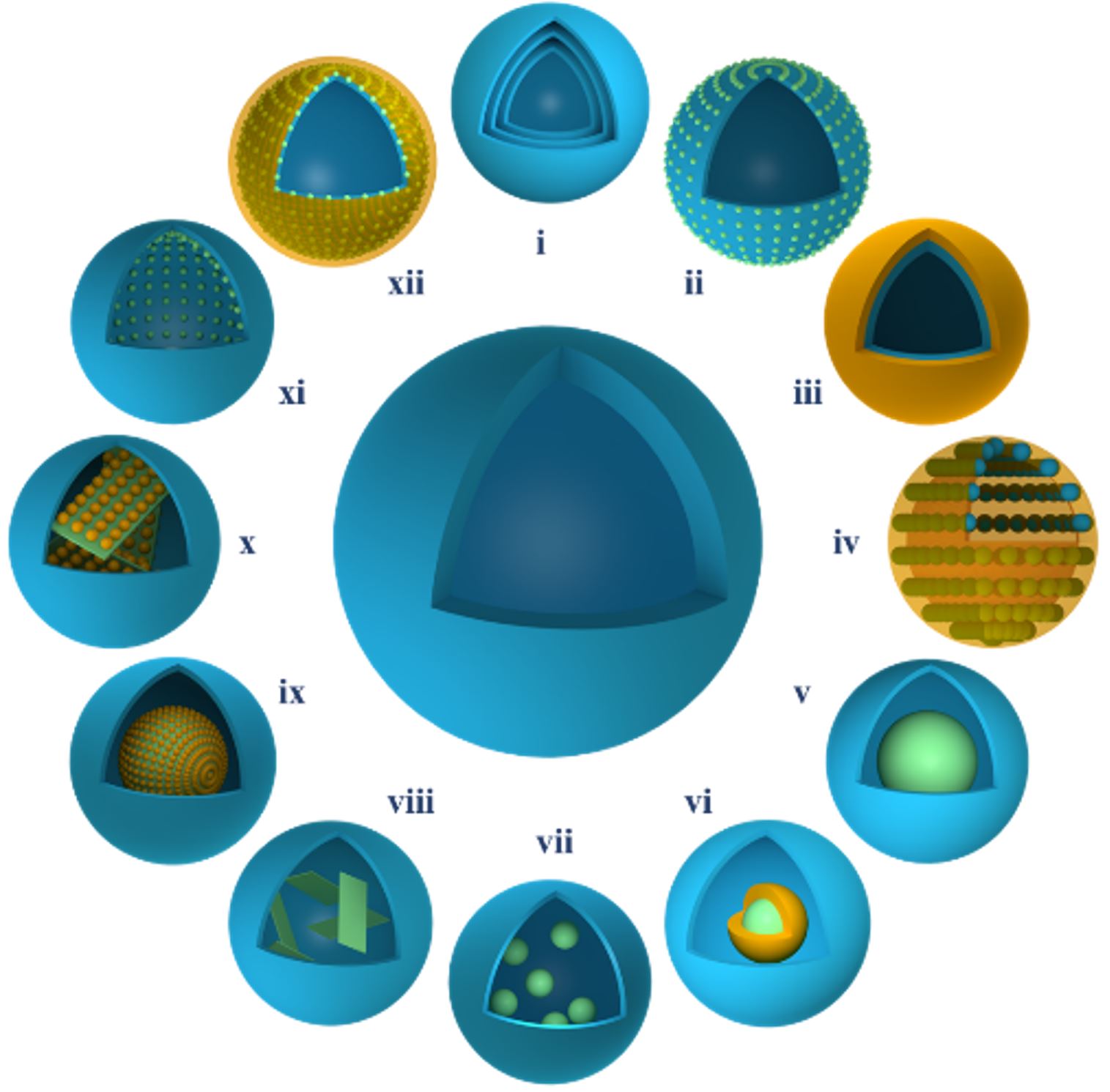
(Figure 1) [5, 6].
|
|
Examples of
Research Activities
|
|
In heterogeneous catalysis, structure and shape of solid
catalysts play an important role in their interactions
with reaction fluids and thus catalytic performance. For
instance, intrinsic nanostructure of catalysts and
related packing in fixed bed reactors will determine the
retention time of gaseous reactants and reaction
intermediates on catalyst surfaces, which in turn
enhances the conversion and selectivity if more
travelling routes and longer travelling paths for
reactants can be created within a catalyst bed, as
reported in our recent investigation on gas-phase
hydrogenation of carbon dioxide [3]. Additionally,
geometric shape of particulate catalysts also impose
significant impacts on their performance, because an
optimal configuration of catalyst can promote transport
processes and thus increase catalytic activity in
fluid-related reactions or environments (Figure 2) [4].
In this regard, a streamline body may represent a
superior geometry since it experiences minimum fluid
resistance. In this latter example, we developed a new
class of integrated nanocatalysts with a streamline body
and tunable chemical compositions. Advantages of these
shape-controlled catalysts were further demonstrated
with some model reactions such as liquid-phase alcohol
oxidation, olefin hydrogenation, and Suzuki-Miyaura
coupling, in comparison with their commonly used
counterparts [4].
|
|
Future
Direction
|
|
Departing from their conventional counterparts, the
state-of-the-art catalysts for heterogeneous catalysis
can be designed and prepared in a controllable fashion
owing to rapid development of nanoscience and
nanotechnology as well as the maturing chemistry of
materials over the past two decades. It is anticipated
that compositional and structural requirements of such
catalysts can be met with a higher level of
sophistication and precision but at a lower cost. As
most of commercial catalysts use expensive metal
elements (such as noble metals), INCs represent a future
form of catalysts to boost atom economy of these
precious metals. However, realization of this
paradigmatic shift for modern catalyst manufacturing
would still require collective efforts from the research
community. We envision that the synthetic architecture
of nanomaterials will become an important field in
future development of state-of-the-art catalytic
materials. We also believe that further integrations of
a single type or multiple types of INCs could lead to
even more powerful “supracatalysts”, achieving
industrial scale applications [1].
|
|
 |
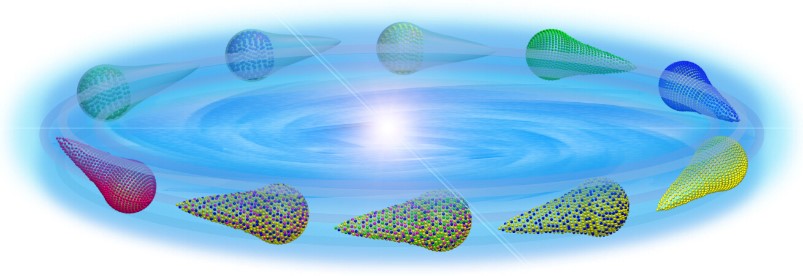 |
Figure 1. Design and synthesis of 12
representative reactor-like integrated nanocatalysts
[6].
|
Figure 2. Streamline-shaped INCs made from
noble metals/organic-inorganic hybrids [4].
|
|
References
|
|
[1] H.C. Zeng, Integrated Nanocatalysts, Acc. Chem. Res.
46, 226-235 (2013).
[2] G.W. Zhan, H.C. Zeng, Integrated Nanocatalysts with
Mesoporous Silica/Silicate and Microporous MOF
Materials, Coord. Chem. Rev. 320-321, 181-192 (2016).
[3] G.W. Zhan and H.C. Zeng, ZIF-67 Derived Nanoreactors
for Controlling Product Selectivity in CO2
Hydrogenation, ACS Catalysis 7, 7509-7519 (2017).
[4] G. Zhan, H.C. Zeng, Smart Nanocatalysts with
Streamline Shapes, ACS Cent. Sci. 3, 794-799 (2017).
[5] G.W. Zhan, P. Li and H.C. Zeng, Architectural
Designs and Synthetic Strategies of Advanced
Nanocatalysts, Adv. Mater. 30, 1802094 (2018).
[6] B.W. Li and H.C. Zeng, Architecture and Preparation
of Hollow Catalytic Devices, Adv. Mater. 30, 1801104
(2018).
[7] G.W. Zhan and H.C. Zeng, Hydrogen Spillover through
Matryoshka-Type (ZIFs@)n-1ZIFs Nanocubes, Nat. Commun.
9, 3778 (2018).
[8] Y.C. Tan and H.C. Zeng, Defect Creation in HKUST-1
via Molecular Imprinting: Attaining Anionic Framework
Property and Mesoporosity for Cation Exchange
Applications, Adv. Funct. Mater. 27, 1703765-73 (2017).
[9] Y.C. Tan and H.C. Zeng, Lewis Basicity Generated by
Localised Charge Imbalance in Noble Metal
Nanoparticle-Embedded Defective Metal–Organic
Frameworks, Nat. Commun. 9, 4236 (2018). |
|

